The past year has challenged us in new and unexpected ways. Yet through it all, we have been inspired by the strength and resilience of our community. We are deeply grateful to be part of West Michigan.
As with so many organizations and individuals, the pandemic upended many well-laid plans here at Van Andel Institute. But it could not stop the progress of scientists and the passion of educators, who have made vital advances despite unprecedented difficulties.
Making strides in cancer
Rather than one disease, cancer refers to a group of more than 100 different diseases, all with one thing in common — uncontrolled cell growth. It represents a complex problem that requires an equally sophisticated approach in order to find new treatments and, hopefully, cures. That’s why VAI scientists tackle cancer from all angles, from studying its molecular underpinnings to investigating its roots in the genetic code to supporting clinical trials designed to find new treatments. Here are some highlights from their efforts in 2020:
- Brian Haab and collaborators are developing a simple, experimental blood test that distinguishes pancreatic cancers that respond to treatment from those that do not. This critical distinction could one day guide therapeutic decisions and spare patients with resistant cancers from undergoing unnecessary treatments with challenging side effects. The experimental test is slated to undergo additional clinical validation. [1]
Read more
- VAI’s Biorepository was awarded a $2.7 million, two-year subcontract from the Frederick National Laboratory for Cancer Research currently operated by Leidos Biomedical Research, Inc. on behalf of the National Cancer Institute to serve as the biorepository for the Cancer Moonshot Biobank study, a national initiative to transform cancer treatment and prevention through accelerated research. [2]
Read more
- The American Association for Cancer Research awarded Van Andel Institute Professor Peter W. Laird, Ph.D., Director’s Scholar Stephen B. Baylin, M.D., FAACR, and Associate Professor Hui Shen, Ph.D., 2020 AACR Team Science Awards for their pivotal roles in the establishment and success of The Cancer Genome Atlas (TCGA), a landmark project that revolutionized our understanding of cancer and that is hailed as an exemplar of scientific collaboration.
Read more
Pursuing progress in Parkinson’s and other neurological disorders
Over the next decade, the incidences of Parkinson’s and Alzheimer’s are expected to increase significantly, reports the Parkinson’s Foundation and Alzheimer’s Association, respectively. Currently, there are no effective ways to slow or stop progression of either disease. At VAI, scientists are delving into the mechanisms that give rise to these diseases in hopes of providing a foundation for new treatments that halt progression and give people more years with fewer symptoms. Highlights from the past year include:
- Can COVID-19 infection increase the risk of developing Parkinson’s disease? That’s the question posed by a commentary published in the journal Trends in Neurosciences, which explores three known case studies of people developing Parkinson’s-like symptoms in the weeks following infection with SARS-CoV-2, the virus that causes COVID-19. While rare, these cases provide important insights into potential long-term implications of infections. The commentary was co-authored by Dr. Patrik Brundin of VAI, Dr. Avindra Nath of the National Institute of Neurological Disorders and Stroke of the National Institutes of Health, and Dr. David Beckham of University of Colorado. [3]
Read more
- A collaborative team between the University of Minnesota Medical School and VAI was awarded $6.2 million for a study that seeks to define the molecular linkages between aging and Parkinson’s disease — an approach for new treatment targets not yet explored by many researchers. The three-year grant comes from the Aligning Science Across Parkinson’s initiative, an international collaborative research effort partnering with The Michael J. Fox Foundation for Parkinson’s Research to implement its funding. The study will combine four labs — Dr. Michael Lee and Dr. Laura Niedernhofer from University of Minnesota, and Dr. Darren Moore and Dr. José Brás from VAI.
Read more
- Rita Guerreiro and Dr. José Brás were awarded a $3.7 million grant from the National Institute on Aging to study the genetic predisposition to Alzheimer’s in the Portuguese population. The study will be the first of its kind in the country. [4]
Read more
- Switching off a molecular “master regulator” may protect the brain from inflammatory damage and neurodegeneration in Parkinson’s disease, according to a study led by Dr. Viviane Labrie. The study is the first of its kind and points to an entirely new avenue for developing therapies that preserve vulnerable brain cells in Parkinson’s disease. [5]
Read more
- A team led by Dr. Viviane Labrie may have solved one of the most puzzling and persistent mysteries in neuroscience: why some people are “right-brained” while others are “left-brained.” The answer lies in how certain genes on each side of the brain are switched “on” and “off” through a process called epigenetic regulation. The findings may explain why Parkinson’s disease and other neurological disorders frequently affect one side of the body first, a revelation that has far-reaching implications for development of potential future treatments. [6]
Read more
Understanding how metabolism fuels health and disease
Metabolism provides fuel for each one of our bodies’ functions, from powering individual cells to ensuring we have the energy to complete our daily tasks. It is intricately linked to health and disease. By investigating how metabolism works and its links to other critical systems, such as the immune system, VAI scientists are charting a path toward a healthier tomorrow. Highlights from 2020 include:
- A newly identified biomarker could help scientists pinpoint which cancers are vulnerable to treatment with biguanides, a common class of medications used to control blood sugar in Type 2 diabetes, reports a study led by VAI’s Dr. Russell Jones. Biguanides, particularly a medication called metformin, have long been of interest to cancer researchers because of their ability to target cellular metabolism, which fuels the growth and spread of malignant cells. [7]
Read more
- The immune system’s ability to marshal specialized cells to fight off infection relies in part on tiny molecules called microRNAs, which act as a release for the “brakes” that keep cells dormant until needed, according to a research team led by VAI’s Dr. Connie Krawczyk. Their findings revealed new insights into the nuts and bolts of immune function and add to a growing body of knowledge that could one day be leveraged to optimize vaccines or immunotherapies for a number of diseases. [8]
Read more
- Significantly reducing dietary levels of the amino acid methionine could slow onset and progression of inflammatory and autoimmune disorders such as multiple sclerosis in high-risk individuals. The findings — an important step toward potential future treatments — must be verified in humans first, said Dr. Russell Jones, who led the study. [9]
Read more
Zooming in on life’s building blocks
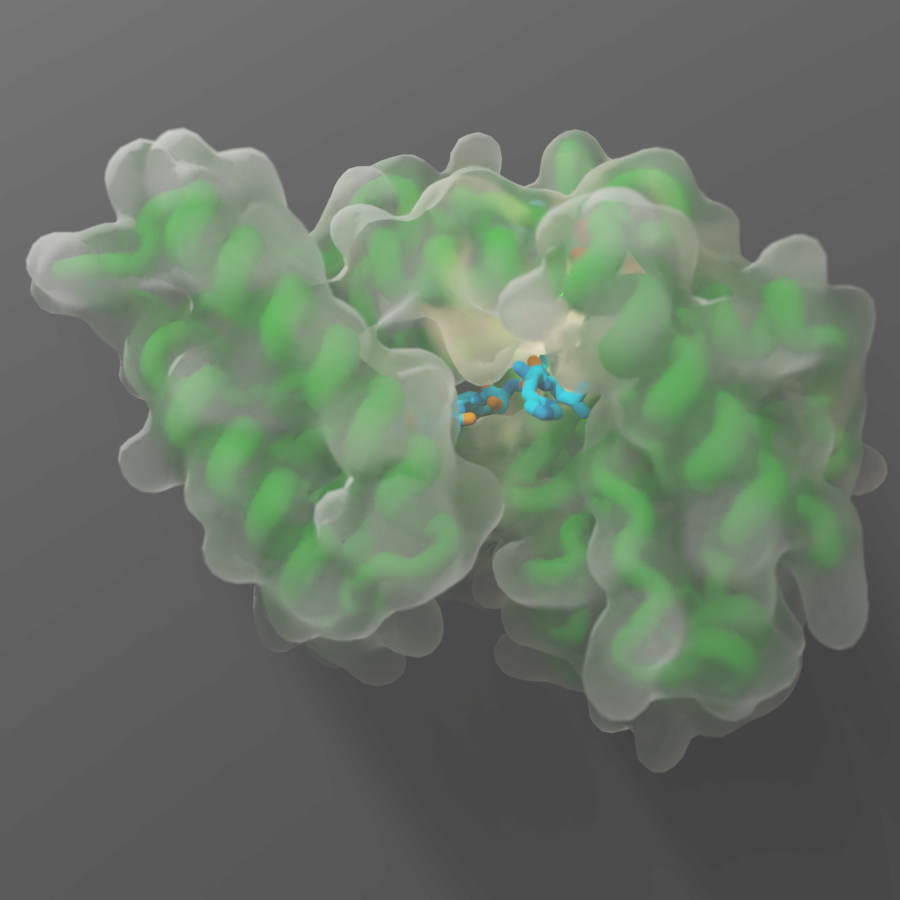
VAI is home to one of the most powerful types of microscopes in the world, which allows our scientists to study life’s tiniest building blocks in exquisite detail. In fact, they are the same types of microscopes used by scientists in the U.S. and abroad to give us detailed glimpses of the virus that causes COVID-19. At VAI, our scientists use this groundbreaking technology, called cryo-electron microscopy, to visualize proteins and other important molecular structures that play important roles in health and disease. Understanding what these molecules look like gives us vital insights into how they work and how they may be targeted by new medications to treat a host of problems, such as cancer, neurological disorders and many others. Here are some highlights from the past year:
- PAC, a class of molecular “gates” that maintain pH balance in cells, which helps keep cells alive and helps prevent stroke and other brain injuries. [10]
Read more
- PANX1, a molecular pathway that plays critical roles in human development, blood pressure regulation, inflammation and cell death. [11]
Read more
- EMC, a molecular “machine” that is responsible for installing signaling proteins into cellular membranes. The findings lay the foundation for potential future therapies for diseases like cancer, Alzheimer’s and cystic fibrosis. [12]
Read more
Education
Student and teacher programs offered by Van Andel Institute for Education transitioned to a virtual format this year, including the implementation of webinars to support teachers as they transitioned to distance learning amid the pandemic. Education also rolled out a free project-based learning unit on how to prevent the spread of the coronavirus as well as mini-lessons on timely topics from respectful debate to the winter solstice.
Read more
Graduate School
Van Andel Institute Graduate School student Maggie Chassé earned a prestigious Predoctoral to Postdoctoral Fellow Transition Award from the National Cancer Institute of the National Institutes of Health. The award, also known as the F99/K00, provides up to two years of financial support for Ph.D. candidates to complete their dissertation research, and up to four years of support for postdoctoral training. 2020 was the second consecutive year a Graduate School student received an F99/K00 award.
Read more
Earlier in the year, the Graduate School welcomed two new team members to the organization: John Vasquez, Ph.D., as director of assessment and professional development, and Allison Roman as director of student support services. These hires, coupled with the construction of a new nearby building to accommodate larger incoming cohorts, signal the growing strength of the organization.
Read more
A number of Graduate School students were asked by Spectrum Health to participate in a COVID-19 literature review early in the pandemic. Many students remained engaged throughout the year in the process, which included reading material and providing feedback on which scientific articles may benefit the physicians treating patients.
Read more
Memorials
Dr. Viviane Labrie passed away in a tragic vehicle accident Aug. 21, 2020. Her ability to look at the world through different lenses allowed her to see old problems in new ways, and ultimately revealed groundbreaking insights with the potential to change lives. Dr. Labrie quickly established herself as a globally recognized leader in her field, holding her first faculty position in Toronto before joining VAI in 2016 as an assistant professor. She rapidly advanced, earning an early promotion to associate professor in 2019. Dr. Labrie earned numerous scientific awards and honors, including highly competitive grants from the National Institutes of Health and Department of Defense. Although early in her career, she already had made groundbreaking discoveries that are transforming the understanding of Parkinson’s and Alzheimer’s diseases, including the revelation that the appendix may be a starting point for Parkinson’s. Her findings led to exciting new avenues of discovery for potential treatments for these diseases, and shed light on the underpinnings of many other conditions, including bipolar disorder, schizophrenia and lactose intolerance. Dr. Labrie made an indelible mark on science, her colleagues and those she mentored, and her impact will be felt for years to come.
Dr. Luis Tomatis, a driving force in bringing VAI to life, passed away Sept. 29, 2020. Originally from Argentina, Dr. Tomatis was an energetic advocate for Grand Rapids. He was influential in creating and maintaining the environment that enabled the Institute to thrive and the Medical Mile to sprout up around us. Dr. Tomatis helped recruit top-tier scientific talent to establish VAI’s first Board of Scientific Advisors and appoint Nobel Laureate Dr. Michael Brown as the board’s first chairman. After serving as VAI’s founding president from 1995–2001, he went on to become the director of medical affairs for the Richard M. DeVos Family. A cardiothoracic surgeon, Dr. Tomatis was a former chief of cardiovascular surgery at Spectrum Health and MSU professor of cardiac surgery. He won numerous awards, volunteered for organizations like the American Heart Association and the Grand Rapids Symphony, and was responsible for arranging for invaluable medical equipment to be sent to his homeland of Argentina.
Dr. Gordon Van Wylen passed away Nov. 5, 2020, at the age of 100. As one of VAI’s original trustees, Dr. Van Wylen was instrumental in establishing the early programs of the Education Institute and created the groundwork for the success of VAI. We continue to see his vision in action through the programs we are developing and implementing today, some 25 years later. A man of immense scientific knowledge, education, experience and integrity, Dr. Van Wylen was an accomplished educator and administrator. He served as dean of engineering at University of Michigan, as Hope College president for 15 years, and as founding trustee and inaugural director of Van Andel Institute for Education. Dr. Van Wylen generously devoted his time and talent to numerous other organizations throughout his life. He was widely regarded as a thoughtful, visionary, kind and respectful leader.
Funding
[1] Research reported in this publication was supported by Van Andel Institute; the National Cancer Institute of the National Institutes of Health under award no. U01CA152653 (Haab and Brand) and award no. U01CA226158 (Haab); the Lustgarten Foundation (Tuveson); and the German Research Foundation (Plenker). The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting organizations.
[2] The project has been funded in whole or in part with federal funds from the National Cancer Institute of the National Institutes of Health under contract no. HHSN261201500003I, Task Order HHSN26100042 through Leidos Biomedical Research, Inc. under subcontract no. 20X062Q. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. government.
[3] Research reported in this publication was supported by Van Andel Institute; the Farmer Family Foundation (Brundin); Division of Intramural Research, National Institute of Neurological Disorders and Stroke of the National Institutes of Health (Nath); and a VA Merit Award (Beckham). The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting organizations.
[4] Research reported in this publication is supported by the National Institute on Aging of the National Institutes of Health under award no. R01AG067426. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
[5] The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Congressionally Directed Medical Research Programs’ Parkinson’s Research Program under Award No. W81XWH1810512 (Labrie). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. In conducting research using animals, the investigators adhere to the laws of the United States and regulations of the Department of Agriculture. Animal research at VAI is conducted in accordance with the National Institutes of Health’s Office of Laboratory Animal Welfare Public Health Service Policy on Humane Care and Use of Laboratory Animals. VAI also is accredited by AAALAC International. This work also was supported by a VAI Innovation Award (Labrie). Labrie also has awards from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health and Michigan State University through the Gibby & Friends vs. Parky Parkinson’s Disease Research Award.
[6] This work was supported by Van Andel Institute. Labrie is supported by the U.S. Army Medical Research Materiel Command through the Parkinson’s Research Program Investigator-Initiated Research Award under award no. W81XWH1810512. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the U.S. Army. Labrie also is supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R21NS112614 and by Michigan State University through the Gibby & Friends vs. Parky Parkinson’s Disease Research Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other granting organizations.
[7] Research reported in this publication was supported by the Canadian Institutes of Health Research under grants MOP-142259 (Jones) and MOP-123352 (Duchaine); The Medical Research Council under grant MC_UU_0015/2 (Hirst); and funding from ImmunoMet Therapeutics. The Goodman Cancer Research Center Metabolomics Core Facility is supported by grants from the Canadian Foundation for Innovation, Canadian Institutes of Health Research and Terry Fox Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting organizations.
[8] Research reported in this publication was supported by the National Sciences and Engineering Research Council (NSERC) under grant nos. RGPIN/2018-06257 and RGPIN/419537-2012 (Krawczyk). Brendan Cordeiro was supported by the McGill Integrated Cancer Research Training Program, the Fonds de la Rescherche du Quebec-Santé and the Canadian Institutes of Health Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting organizations.
[9] Research reported in this publication was supported by the Multiple Sclerosis Society of Canada (Larochelle) and the Canadian Institutes of Health Research under award nos. MOP-399061 (Witcher) and MOP-142259 (Jones). The content is solely the responsibility of the authors and does not necessarily represent the official views of any funding organization.
[10] Research reported in this publication was supported by Van Andel Institute; McKnight Scholar Awards in Neuroscience (Du, Qiu), Klingenstein-Simons Scholar Awards (Du, Qiu); Sloan Research Fellowships (Du, Qiu); the National Institute of General Medical Sciences of the National Institutes of Health under award no. R35GM124824 (Qiu); the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award no. R01NS118014 (Qiu), R01NS112363 (Lü) and R01NS111031 (Du); the National Heart, Lung and Blood Institute of the National Institutes of Health under award no. R56HL144929 (Lü); a Pew Scholar in Biomedical Sciences award (Du); and the American Heart Association under award no. 20POST35120556 (Ruan) and 18PRE34060025 (Osei-Owusu). The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting organizations.
[11] Research reported in this publication was supported by Van Andel Institute.
Lü is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under grant no. R56HL144929. Du is supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under grant no. R01NS111031, a McKnight Scholar Award, a Klingenstein-Simons Scholar Award and a Sloan Research Fellowship in neuroscience. Ruan is supported by an American Heart Association postdoctoral fellowship for his study on the PANX1 channel. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other granting organizations.
[12] Research reported in this publication was supported by Van Andel Institute and the National Cancer Institute of the National Institutes of Health under award no. CA231466 (Li). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The post A look back at 2020 appeared first on VAI.